https://botanixpharma.com/wp-content/uploads/Copy-of-BOT-website-announcement-2.png
850
1600
Haley Chartres
/wp-content/uploads/botanix-logo.png
Haley Chartres2025-11-20 00:08:032025-11-20 00:08:04AGM Chairman’s Address is now available
https://botanixpharma.com/wp-content/uploads/Copy-of-BOT-website-announcement-2.png
850
1600
Haley Chartres
/wp-content/uploads/botanix-logo.png
Haley Chartres2025-11-20 00:08:032025-11-20 00:08:04AGM Chairman’s Address is now available
Latest news
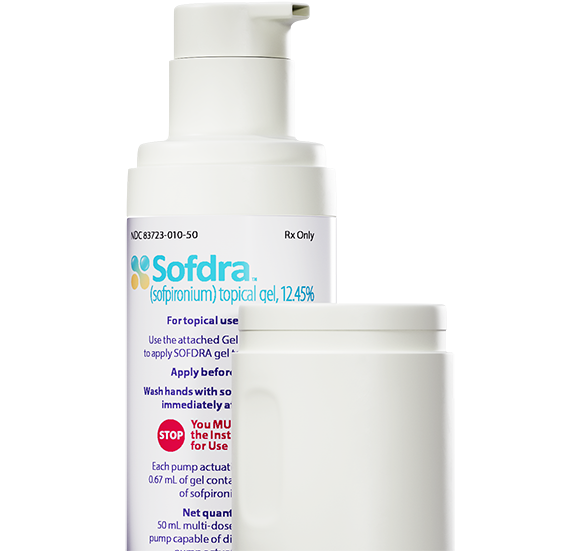
Sofdra™ (sofpironium) topical gel, 12.45% is now available in the US
Our lead dermatology product, Sofdra, is approved for use by the US Food and Drug Administration (FDA).
Innovators and commercial leaders in dermatology
Here at Botanix, we are at the forefront of dermatological innovation. With a world-class team and significant capital, we are committed to addressing common yet underserved skin diseases and infections.
As an ASX-listed company, we are in a prime position to develop and commercialise groundbreaking treatments across broad markets, all while adhering to the highest clinical standards set by the US FDA.
Check out our latest news