BTX 1801 Clinical Development Update
Botanix Pharmaceuticals has today released a comprehensive update and presentation on imminent plans for the BTX 1801 antimicrobial platform program.
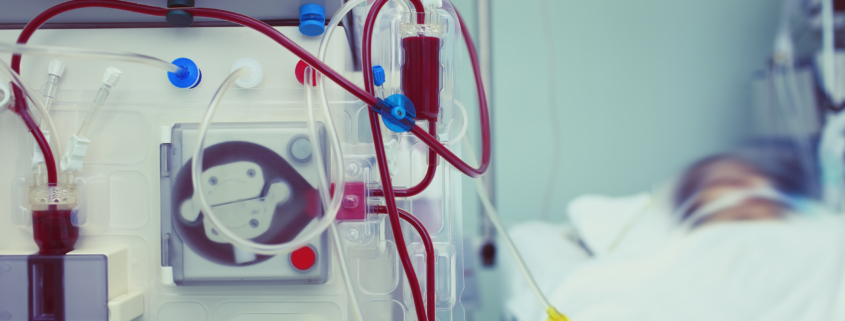
The next phase of development for BTX 1801 will focus on targeting the nasal decolonisation of Staph aureus in haemodialysis patients, which represents a significant market opportunity to prevent bloodstream infections in these patients.
Dialysis largely replicates the functions of the kidneys in patients with chronic kidney failure, with dialysis taking over the key functions of the kidneys (including filtering and removing waste materials from the body). Haemodialysis patients undergoing ongoing dialysis regularly (e.g. three to five times per week), are at a high risk of bloodstream infections, due to their treatment requiring frequent use of catheters which in the first year are routinely central lines with direct access to the heart.
Our team is energised by the opportunity to develop a novel approach for removing sources of bacteria to prevent bloodstream infections in haemodialysis patients – which is desperately needed. Our prior work to examine the unique bactericidal mechanism of action of synthetic cannabidiol provides great confidence for this program too.